E
Velandy Manohar, MD.
Distinguished Life Fellow, Am. Psychiatric Assoc.[APA]
Medical Director, Aware Recovery Care-CT. [ARC], President, ARC In-Home Addiction Treatment, PC
Member- CT. State Medical Society Committees – 1. Bioethics, 2. Quality of Care,3. Disaster Preparedness.
Member- Governance Committee of Consumer Advisory Council of Office of Health Strategy-CT
Founding member- Psychotherapy Caucus of APA [Past Steering Comm member]
Founding member- Community Resilience Collaborative- Mx County-CT [Effects of Childhood Trauma.]
Member- 1. Advisory and Review Board- Whiting Forensic Board- DMHAS-CT,2. Medical Advisory Board- Commissioner of Motor Vehicles- [DMV -CT] 3. Hearing Panel- CT. Medical Examining Board-[DPH]
CT. Multi-Cultural Health Partnership- Nancy Berger Member Award- 2012.
American Health Council- Best in Medicine- 2018
IHAT Addiction Institute- First Impact Award Recipient- 2019
I
https://www.sciencenews.org/article/coronavirus-testing-diagnostic-covid19-united-states
What you need to know about coronavirus testing in the U.S.
Testing remains limited in the country
By Tina Hesman Saey
MARCH 6, 2020 AT 6:24 PM
U.S. government officials say a million promised tests for diagnosing coronavirus infections will soon be in the mail. But that still leaves many state and local laboratories without the ability to test for the virus, crucial for curbing its spread around the country.
Some states have developed their own tests. Clinical testing companies are now joining the ranks. LabCorp announced March 5 that physicians or other authorized health care providers could already order its test. Quest Diagnostics announced the same day that the company will also offer commercial tests as soon as March 9, pending U.S. Food and Drug Administration reviews. Participation of those two commercial laboratories could greatly expand testing capacity in the United States.
But for now, “we still find ourselves as a country with pretty limited capacity to test,” says Michael Mina, an epidemiologist at the Harvard T.H. Chan School of Public Health in Boston.
Here’s what you need to know about coronavirus testing in the country.
What’s the status of testing?
As of March 11, 81 state and local public health laboratories in 50 states and Washington, D.C., have successfully verified COVID-19 diagnostic tests and are offering testing, according to the U.S. Centers for Disease Control and Prevention. But even states that have tests may have only a single kit, containing enough material to test just 700 people, Mina says.
As of March 13, roughly 14,200 tests have been conducted nationally, according to an analysis from the Atlantic.
That’s up from the 1,583 people that had been tested at CDC, as of March 5. Contrast that with the United Kingdom, where 20,388 people had been tested as of March 6. At that point, only 163 cases of COVID-19 had been detected there.
As of March 13, U.S. health officials have reported over 1,885 coronavirus cases across 47 states and Washington D.C., including 41 deaths. More cases can be expected as testing ramps up, experts say.
As more cases are found, health officials will need to test contacts of people who carry the virus, and other ill people in affected communities may demand tests, all escalating the need for more tests.
Vice President Mike Pence told reporters March 5, “We don’t have enough tests today to meet what we anticipate will be the demand going forward,” according to CNN. But having companies’ tests in the mix could help testing ramp up.
To get a more complete picture of how widespread the virus is in the United States, “we’re going to need millions and millions and millions of tests,” said Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases in Bethesda, MD., during a CNN town hall on March 5.
How does a test work?
Health professionals will swab a person’s nose or throat, collect phlegm coughed up from the lungs, or squirt liquid into the nose, throat or lungs and collect the liquid again for testing. Neither Quest nor LabCorp will collect such specimens, but doctors or other health providers may send samples to the labs for testing.
Then, those samples are analyzed in a laboratory, where technicians must extract and purify the virus’s genetic material from the mucus, cell debris and other stuff in the samples. “That sample preparation process is usually the biggest bottleneck [in testing],” says Brent C. Satterfield, founder and chief scientific officer of Co-Diagnostics, a company based in Salt Lake City and Gujarat, India, that has developed its own coronavirus test. That test can be used clinically in Europe but has not yet been approved for use in the United States, although other labs can use components of the company’s test to build their own diagnostic tests. [It detects only one of 3 genes. The US Test detects two of the Genes. VM
See all our coverage of the 2019 novel coronavirus outbreak
All of the coronavirus tests being used by public health agencies and private labs around the world start with a technique called polymerase chain reaction, or PCR, which can detect tiny amounts of a virus’s genetic material. SARS-CoV-2, the virus that causes COVID-19, has RNA as its genetic material. That RNA must first be copied into DNA. “That’s a lengthy part of the process, too,” says Satterfield, adding 15 to 30 minutes to the test.
After that, the PCR can begin. The process makes millions to billions of copies of selected segments of DNA. In the case of the coronavirus, the CDC’s original test scanned for three of the virus’s genes, but now tests for two. The World Health Organization’s test, developed by infectious disease researcher Christian Drosten at the Charité – Universitätsmedizin Berlin and colleagues, tests for three genes but is a bit different than the CDC tests. The PCR step typically takes 45 minutes to an hour, Satterfield says.
Some assays give instant yes or no readings, but others may also take time to analyze. Altogether, it may take about three hours to complete a test, Satterfield estimates.
Can my doctor do the test?
PCR tests are not simple enough to do in a doctor’s office.
In the United States, a doctor is now allowed to decide if a test is warranted and collect the sample, but then must ship the sample off for other trained professionals to prepare and test.
Testing was initially limited to only those people with symptoms and a travel history to an affected area or contact with a known case. On March 4, the CDC relaxed some restrictions on who can get tested. “People still have to be sufficiently sick and have failed a flu test” in order to qualify for coronavirus testing, Mina says.
In some states, the positive test results are called presumptive positives until the CDC can confirm them. In those cases, the final official result may take days. LabCorp estimates that it will take three to four days to return results to physicians.
Why can’t the virus be tested for like influenza?
Many doctors’ offices can do a rapid influenza test. But those flu tests don’t use PCR, Satterfield says. Instead, they detect proteins on the surface of the influenza virus. While the test is quick and cheap, it’s also not nearly as sensitive as PCR in picking up infections, especially early on before the virus has a chance to replicate, he says. By the CDC’s estimates, rapid influenza tests may miss 50 percent to 70 percent of cases that PCR can detect. The low sensitivity can lead to many false negative test results.
Flu tests also aren’t as specific for a particular virus strain as PCR is. About 5 percent to 10 percent of the time, flu tests may mistake a different virus for the flu, creating a false positive result. “Specificity is a big deal when you’re testing large numbers of people who aren’t expected to be positive,” Satterfield says. “If you’re going to test in one of the states that doesn’t have a coronavirus outbreak right now, with a specificity of 90 percent, 10 out of every 100 people are going to show up positive even though the coronavirus isn’t there yet.”
“Accurate diagnosis is a very high imperative for this [coronavirus],” Satterfield says.
An additional benefit of a PCR test is that it may be able to detect viruses earlier in an infection than a flu-style test can, he says, perhaps not in the first day, but a couple of days into an infection when the virus is replicating strongly, but the body’s immune system hasn’t yet begun to fight and produce symptoms.
“In every infectious disease I know of, that is the most contagious period for a person; the point in time when the virus has multiplied to its maximum capacity and the body has not yet started to rein in on it,” Satterfield says. Being able to identify people in that period and isolate them from others could help curb the spread of the disease.
Why was broader testing delayed in the first place?
Delays started with a manufacturing flaw in the CDC’s original PCR test, which caused components that detect one of the three targeted viral genes to not work properly, the health agency says.
Those woes sound like user error to Co-Diagnostics’ Satterfield. “A lot of what they are seeing is probably due to inconsistent use in the field,” he says. “Tests that work phenomenally well in the lab, when they are sent to the field, sometimes just don’t work the same,” he says.
Co-Diagnostics’ test also uses PCR but tests for only one gene versus three. “Sometimes the more complexity you have in a test, the more things you have that can go wrong,” Satterfield says.
Some delays in getting to test off the ground came from emergency measures enacted by the FDA, Satterfield says. Normally, big medical testing labs, such as state health labs and companies like LabCorp and Quest Diagnostics, are allowed to develop and validate their own tests. But when the coronavirus was declared a public health emergency on January 31, labs needed “emergency use authorization” before using their tests to diagnose cases. Even the CDC had to get permission to use its test. But on February 29, FDA announced that labs could devise their own tests and use them clinically while waiting for the agency to review their applications. “FDA does not intend to object to the use of these tests for clinical testing while the laboratories are pursuing an EUA,” the agency said in a statement.
“It looks like there were some pretty large blunders that led to some serious delays,” says Mina, the epidemiologist at Harvard. “Instead of reducing the amount of testing at the start of an epidemic … we should have been expanding it as quickly as possible and calling for all hands-on deck,” he says.
Those delays and the initial limitations on who could be tested may have allowed some cases to slip through the cracks and start community outbreaks in Washington and California.
Where can I get tested?
It will vary from place to place. If you have symptoms of COVID-19 — fever, dry cough and often fatigue — contact your doctor or local or state health department for more information. Do not go to the emergency room for testing, officials say.
II
https://towardsdatascience.com/statistics-and-unreliable-tests-coronavirus-is-difficult-to-contain-e113b5c0967c
THE 2020 NOVEL CORONAVIRUS OUTBREAK | THOUGHTS ON PROBABILITY AND STATISTICS
Bayes’ Rule, Unreliable Diagnostic Testing, And Containing COVID-19
How false negatives in diagnostic testing lead to the release of infected people, motivate extreme containment measures have been implemented, explain why official figures are too low.
The COVID-19 outbreak explained with Bayes’ Rule.
Andy Chen, Feb 19,2020
Wuhan coronavirus. Novel coronavirus. COVID-19.
We are currently in February 2020. Over the past month, a deadly virus has been spreading throughout China and the world, sending the infected to the ICU and trapping others in their homes. As authorities try to manage this crisis, they face the challenging issue of containment — sending the infected to quarantine, while allowing the non-infected to go free.
Here is the scenario. You have a cough and a fever. There is a chance that you have caught COVID-19 — the virus spreading throughout the world. You don’t know what this chance is, and you don’t want to take chances, so you seek advice from your doctor.
The issue from the authority’s perspective is different. You want treatment, but the authorities need to contain the spread of the virus. From their point of view, there are 4 main outcomes of your visit to the doctor.
1. If you are infected and diagnosed with coronavirus, they will quarantine you for the public benefit.
2. If you are not infected but you are diagnosed with coronavirus,[False Positive] they will wrongly quarantine you, causing you inconvenience. The public will suffer no major harm, but the authorities will have to expend a small amount of resources.[ To try to track down contacts and test them]
3. If you are infected and not diagnosed with coronavirus, [False Negative] they will wrongly release you, causing you to spread the virus. This puts the public in grave danger of an outbreak.
4. If you are not infected and not diagnosed with coronavirus, they will rightly release you and save some resources.
The two mistakes that the authorities can make is scenario 2 and 3. Scenario 2 is a minor inconvenience (if not done too often), but scenario 3 is the major issue which can cascade into a larger outbreak, even if only done once. If an outbreak occurs, they will have to do contact tracing for possibly hundreds of people, given the contagiousness and lethality of coronavirus. This will be extremely costly for them, so their primary interest is in minimizing the probability of the third scenario.
Thus, the authorities need to make an accurate diagnosis, so that they can avoid releasing the infected and quarantining the non-infected. To achieve this, the authorities first make an initial assessment of all suspected infections, whether they are patients at the medical clinic, or travelers from places with active outbreaks.
Thus, the authorities need to make an accurate diagnosis, so that they can avoid releasing the infected and quarantining the non-infected. To achieve this, the authorities first make an initial assessment of all suspected infections, whether they are patients at the medical clinic, or travelers from places with active outbreaks.
Initial Assessment of a Patient
There are clues which will hint at a COVID-19 infection.
• Location: there will be different probabilities of infection for people living in different places. People near the epicenter are more likely to be infected. People living near popular tourist destinations are more likely to be infected than people from isolated places like rural Alaska.
• Travel history: travelers from places near the epicenter or other outbreak locations are more likely to have the infection (hence, why travelers are screened).
• Social contacts: people who have close contact with the infected/those at risk of infection are more likely to be infected themselves.
• Symptoms: people who have symptoms characteristic of coronavirus, such as fever, cough, and shortness of breath are more likely to be infected.
• Imaging: people who show imaging features of coronavirus on an x-ray or CT scan are more likely to be infected.
Not all of these clues will be immediately available. Authorities at the airport can only screen people for temperature and identify travel history if needed. A doctor will know about the symptoms disclosed by the patient, but it is on the doctor to take the initiative to find and link the clues together. And an asymptomatic case may not have any clues.
You go to the doctor, and the doctor asks you a series of questions. You tell the doctor about your cough and fever. The doctor is suspicious and builds their own belief of whether you are infected or not. Their belief is strong enough to justify a coronavirus test, so they order you to take this test.
The Probability and Statistics Behind Diagnostic Testing
After gathering enough clues, a doctor may suspect coronavirus. To confirm this suspicion, the doctor will order a diagnostic test.
Misconceptions about what the diagnostic test result means
A diagnostic test is performed by collecting samples from your body (e.g. mucus in the back of the nose) and looking for presence of the virus in those samples. It seems simple enough, and people have a lot of faith in science. This may lead people into making this first mistake.
Incorrect interpretation: a positive result means a patient has novel coronavirus, while a negative result means that a patient does not.[ It is not all the Straight forward.VM]
This is not true, because the test is not always reliable. There are many reasons why a test may give a misleading result:
• A patient in the very early stages of an infection may not excrete a detectable amount of virus.
• The virus itself may only exist deeper inside the body, hence being inaccessible by a swab test.
• There may have been accidental contamination of the sample. [Including the possibility of cross contamination and even mislabeling at various points along the pat taken by the sample from the time it is collected, and the results are reported. VM]
In general, the people who make the testing kits will specify the reliability of the test.
Suppose that a company now markets their test as “90% accurate”. This can lead to another common mistake.
Incorrect interpretation: for a 90% accurate test, a positive result means 90% chance of being infected, and a negative result means 90% chance of not being infected.
This interpretation is also not true, but is actually surprisingly common in the medical community— the great psychologist Gerd Gigerenzer shows how doctors misinterpret the results of mammogram results article.
Bayesian probability explains what the diagnostic test really means
The correct way to evaluate a diagnostic test requires thinking in terms of Bayesian probability. To put it simply, Bayesian probability involves having a prior probability and then using new information to update it. In terms of diagnostic testing, the prior probability is the doctor’s belief about whether the patient is infected or not. The test result is used as information, and this changes the doctor’s belief.
We can formulate the reliability question in terms of math equations.
Prior probability
The initial probability of infection, which is based on the doctor’s judgement.
Posterior probability: how reliable is a positive result?
The probability of infection is conditioned on a positive result. The positive result is information.
Posterior probability: how reliable is a negative result?
The probability of (no) infection is conditioned on a negative result. The negative result is information.
What we need to do now is to connect the doctor’s initial belief with the final belief. To do this, we use Bayes’ rule, which can easily be derived using basic factors about conditional probabilities.
The denominator is expressed like this.
We now have two new unknown probabilities.
1. The probability of a positive result given that a patient is infected. This number should be high — infected patients should be getting positive test results.
2. The probability of a positive result given that a patient is not infected . This number should be low — non-infected patients should be getting negative test results.
These two probabilities are actually measuring of a test’s reliability, and they can be expressed in terms of two quantities: sensitivity and specificity.
Sensitivity
• Probability of a positive result given infection.
• The test is “sensitive” to the presence of coronavirus. If the coronavirus is present, the test will detect it.
• Ideally, close to 100%.
Specificity
• Probability of a negative result given no infection.
• The test is “specific” to coronavirus. If there is no coronavirus infection, the test will not detect anything, and returns negative.
• Ideally, close to 100%.
I should remind you here that I can express probabilities in terms of complements. Infection and no infection are mutually exclusive. Similarly, a positive and a negative result are also mutually exclusive. This means that our calculations will use these two equations, where A and B are events such as “the patient is infected”, or “the test result is positive”.
The probability of A not occurring is one minus the probability of A occurring.
Given that B has occurred, the probability of A not occurring is still one minus the probability of A occurring.
The probability of infection given a test result
We now have all the tools we need to interpret a test result
• Prior probability — initial belief
• Posterior probability — final belief
• Bayes’ rule — connects initial and final belief
• Sensitivity and specificity — allows us to do computations using Bayes’ rule
Combining these, we get
We now know how to incorporate the information from a test result to change an initial belief into a final belief. Next, we look at diagnostic tests with different levels of reliability and see how their results might affect the beliefs of doctors.
The Application of Diagnostic Tests in Medicine
You followed the doctor’s advice and got a diagnostic test. The doctor now receives a positive or negative result for coronavirus and now needs to make a decision:
1. Prescribe any necessary medication and let you go.
2. Order you to be quarantined [or not. VM]
Decision Making in Medicine — Reducing Uncertainty And Taking Acceptable Risks
To decide when to treat, the doctor needs to have confidence in a diagnosis.
In general, medical practice, a doctor can make two mistakes:
1. You are not infected, but the doctor decides that you are, and gives treatment.
2. You are infected, but the doctor decides that you aren’t, and does not give you the proper treatment / lets you go.
In order to avoid these mistakes, the doctor orders a test. They had a prior belief, and the test is used as information to reduce uncertainty. Once the doctor has enough certainty, the doctor can make a recommendation.
________________________________________
How much uncertainty can be reduced by a test?
We first look at a reliable test. Consider a test that has sensitivity and specificity at 98%. We show what the prior and posterior probabilities of infection are, given a positive or negative result.
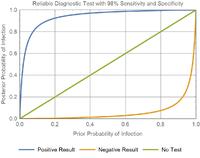
• If you think the probability of someone being infected is 80%, then a positive result takes it to near 100% (near certain infection), while a negative result reduces the probability to under 10% (unlikely infection).
• Similarly, if you think that the probability of someone being infected is only 20%, then a positive result increases it to over 90% (likely infection), while a negative result takes it to near 0% (infection is nearly impossible).
• Any initial probability which is greater than 10% increases to a higher probability (>80%) after a positive result.
• Any initial probability which is less than 90% decreases to a lower probability(<20%) after a negative result.
• Since the test is able to change a large range of initial probabilities into final probabilities near 0% and 100%, it resolves the uncertainty of whether a patient is infected or not. Hence it is a reliable diagnostic tool.
The Ideal test
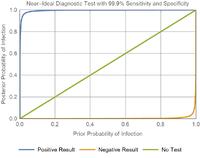
• The orange curve (bottom, for negative result) is very close to the x-axis, which would mean that a negative result virtually guarantees a zero probability of infection.
• The blue curve (top, for positive result) is straight up the y-axis and right, which would mean that a positive result virtually guarantees a certain probability of infection.
________________________________________
Diagnostic tests are not perfect — bad luck exists, medical decisions are always risky propositions, fraught with decisional challenges
If the doctor has a reliable test, then a prior belief of 50% can be reduced to less than 5%, or some other very low figure. Unfortunately, these tests are not perfect. There is still a non-zero probability of actually being infected, so the doctor can make a mistake out of sheer bad luck.[i.e. False negative]
How much risk is tolerable?
[In a sense as fiduciaries of the American People Medical and Civil Authorities We must ascertain the public acceptance of degree of risk Medical Economic and Social costs fo flattening the curve as we pursue the goals to restore stability and wellbeing all across the board at many levels and in many domains over time concordant to the Public will. This War against COVID -19 must not end like the VN war because of the erosion of the Public will to support the positions advocated by our Govt. The consequences for the American Civilization may well be catastrophic, and hard to recover from.VM][In a sense as fiduciaries of the American People Medical and Civil Authorities We must ascertain the public acceptance of degree of risk Medical Economic and Social costs fo flattening the curve as we pursue the goals to restore stability and wellbeing all across the board at many levels and in many domains over time concordant to the Public will. This War against COVID -19 must not end like the VN war because of the erosion of the Public will to support the positions advocated by our Govt. The consequences for the American Civilization may well be catastrophic, and hard to recover from.VM][In a sense as fiduciaries of the American People Medical and Civil Authorities We must ascertain the public acceptance of degree of risk Medical Economic and Social costs fo flattening the curve as we pursue the goals to restore stability and wellbeing all across the board at many levels and in many domains over time concordant to the Public will. This War against COVID -19 must not end like the VN war because of the erosion of the Public will to support the positions advocated by our Govt. The consequences for the American Civilization may well be catastrophic, and hard to recover from.VM]
[This is key.VM]
Because the doctor ultimately has to make a decision, even under uncertainty, the doctor has to decide what is an acceptable risk.
When risk is unacceptable:
• Near-certainty is required before starting a harsh treatment with irreversible side effects.
• A very low probability of having a lethal infectious disease is required before the patient is released.
Where risk is more acceptable:
• Less certainty is needed if the condition is mild and not life-threatening, and the treatment does not have permanent effects.
• Less certainty is needed if the patient is in immediate mortal danger and requires medical intervention as soon as possible (the potential harm of treatment is less than the harm of no treatment).
Decision Making Without Reliable Diagnostic Tests
(From here, we switch focus from general medicine, back to the coronavirus outbreak.)
We’ve established that a doctor needs to resolve uncertainty using a diagnostic test before they can have the confidence to take an acceptable risk.
However, what happens if the diagnostic test is not reliable?
________________________________________
We try to use realistic values. Many flu tests have a specificity of around 90–95%. Keeping in line with this, I assume a 90% specificity (feel free to correct me if you have better information).
I assume a 40% sensitivity. This turns out to be close to the reported sensitivity for COVID-19 coronavirus tests. VM
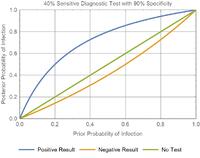
Suppose that a patient lives in an area which recently had an outbreak and is now presenting to the medical clinic with symptoms of coronavirus. If the doctor deems the probability of a COVID-19 infection to be 50% (likely infection), and orders this unreliable test for the patient:
• A positive result increases the probability to 80% (likely infection)
• A negative result decreases the probability to a value to 40% (possible infection).
• 40% probability of infection is still too high. Given that the virus is contagious and lethal, releasing the patient is an unacceptable risk.
With the reliable test:
• A 50% probability of infection can be decreased to 2% with a negative result.
• A COVID-19 infection can potentially be ruled out (if 2% is acceptable risk).
Using a single unreliable test, a patient‘s probability of infection cannot be reduced to an acceptable level, regardless of a positive or negative result.
The unreliable test cannot rule out infection.
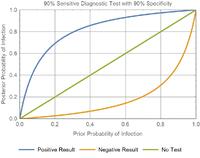
What happens as a test gets less and less sensitive? Negative results are less meaningful — the orange line (negative result) quickly approaches the straight green line (no test performed).
No decision making (for epidemics) without reliable diagnostic tests
Now imagine a scenario where people are quarantined until the authorities are confident that they are not infected. If the test is unreliable, then it cannot prove that there is no infection. In other words, the test has no consequences on decision making — the patient will remain in quarantine either way. [A very serious ethical and social problem.VM]
With contagion potential, unreliable tests have no influence on decision making.
Mistakes happen when test reliability is not understood
What if a doctor does not know the reliability of a test?
A doctor would understand that COVID-19 is highly contagious and lethal, and thus, their level of acceptable risk would be very low.
Suppose they mistook an unreliable test for a reliable test. Their initial belief of 50% probability would drop to an actual 40% probability of infection, while in their mind, it would be 2% because they assumed that the test was reliable. If they went on to release the patient, this would potentially have disastrous consequences.[ To their patients, their contacts and the community in general.VM]
This is why the tools used in the clinic are backed by research. Doctors need certainty about what their tools are and aren’t capable of.
Checking multiple times with multiple tests.
If a doctor knows about the unreliability of a test and trusts their own judgement, then a single negative test result will not convince them. The doctor will dismiss the test result as an error.
How many negative test results does it take to convince the doctor?
Assume that if the probability is reduced to less than 5%, then the doctor will be sufficiently convinced that there is no infection.
After a clinical examination, a doctor believes that a patient has 50% probability of having COVID-19. He knows that the diagnostic test is unreliable, with 40% sensitivity and 90% specificity, and so he orders a series of diagnostic tests.
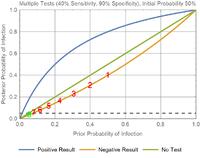
Every diagnostic test returns a negative result. The initial probability is 50%, and each test decreases the probability.
50% → 40% →30.8% → 22.9% → 16.5% → 11.6% → 8.0% → 5.5% → 3.7%
In the graph, you can see that the green point has 5.5% prior probability and ends up with 3.7% posterior probability, which is past the grey threshold of acceptable risk.
It takes 8 consecutive negative results to convince a doctor who starts with a probability of 50%. Note that if it takes X tests starting from probability Y, then it will take at least X tests for any probability greater than Y. Any patient with an initial probability of infection greater than 8% will require at least 2 tests to rule out an infection.
Is this a feasible solution?
The Role of Unreliable Tests in the COVID-19 Outbreak
The COVID-19 Diagnostic Tests Are Unreliable — Low Sensitivity
________________________________________
Update (Feb 21): I feel the need to clarify something.
There may have been something lost in translation. Other experts claimed that it is not 30–50% sensitivity, but 30–50% of suspected patients who test positive. This means that the test itself isn’t flawed.
I’m not sure what that means for the other suspected patients who test negative — are they infected or not? I thought they were, since they would have been suspicious enough to have received a test in the first place, and the authorities ended up having the confidence to add “clinical confirmations” as a category.
If they aren’t actually infected, then either the number of tests is lower than reported, or the proportion of infections among tested people is much lower than 90–100% (probably around ~50%).
But if they are infected, then that could mean that the virus isn’t showing up in samples. In this case, the sensitivity problem remains relevant, and is fixed with better sampling, rather than a more reliable test.
________________________________________
For the current outbreak of coronavirus, there have been reports of negative results for people who are actually infected — a false negative.
On the patients being tested with nucleic acid tests (NATs), used to diagnose COVID-19.
“Not all of those infected by it return positive NATs”.
“Even patients who definitely have the disease only come back positive 30 per cent to 50 per cent of the time.”
“Testing throat swabs (from potentially infected people) also returns a lot of false negatives.” — Professor Wang Chen, director of the Chinese Academy of Medical Sciences (source: Caixin Media, a Feb 8 article.)
The experts themselves are saying that the tests are not sensitive enough — probability of a positive result for an infected patient is too low.
The tests being described by these experts are similar to the unreliable tests that I have been using as an example.
How is the containment of COVID-19 progressing?
Releasing or not accepting patients with a negative result
“Some patients whose CT scans clearly showed signs of viral infection but whose NATs tested negative were “released” back into their communities due to a shortage of hospital berths.” — anonymous doctor in the imaging department of a major Wuhan hospital.
(Source: Caixin Media)
“Though her doctor was almost certain she was infected with the virus, a throat-swab test she had taken came back negative, which meant the facility wouldn’t take her.”
(Source: Wall Street Journal)
While these events are driven more by a lack of resources, it is sad to see patients being rejected because of low-sensitivity tests.
Ordering multiple tests, not releasing patients with a negative result
Wuhan’s top Communist Party official, Ma Guoqiang, cast doubt on the swab test on Monday, urging those with negative results to try again to be sure. “One needs to wait one day later, to get another negative, in order to rule out [the virus],” he said.
(Source: Wall Street Journal)
“Her clinical features are very typical of the coronavirus infection. We didn’t discharge her, because we still have enough beds and can’t run the risk of letting her infect someone else. And on the fifth try, the result finally came back positive.” — Bin Song, director of the radiology department at Huaxi Hospital in Chengdu.
(Source: Interview with Al Jazeera)
While multiple tests can provide confidence if a positive result is eventually reached, it has its costs, and the patients should have been quarantined and treated either way (since disconfirmation is nearly impossible).
Do tests even add any valuable information in Wuhan patients?
For a 40% sensitive, 90% specific test, we just saw that it takes 8 consecutive negative results to reduce a 50% probability of infection to less than 5% (where very low probabilities are required to reduce the risk of contagion). We also saw that it takes 2 consecutive negative results to reduce 8% to less than 5%. What is the probability of a person in Wuhan being infected, if they:
• Have already shown signs in a CT scan? (surely >50% probability?)
• Have a cough/fever? (surely >8% probability?)
• Have entered any hospital?
It seems like testing for the purposes of ruling out infection is not a feasible solution in an active
• Are related to someone who has been infected?outbreak zone. There are simply not enough resources to test everyone multiple times.
This may partially explain why…
… Authorities have already implemented large-scale containment measures — they assume that everyone is or will be infected.
Mandatory quarantine.
Velandy Manohar, MD

